Here’s a breakdown of how the body regulates acid-base balance and what happens when it’s disrupted:
1. Sources of Acids and Bases
- Acids are produced mainly as by-products of metabolic processes. For example:
- Carbon dioxide (CO₂) is a major byproduct of aerobic metabolism and combines with water to form carbonic acid (H₂CO₃), which can dissociate into hydrogen ions (H⁺) and bicarbonate (HCO₃⁻).
- Lactic acid is produced during anaerobic respiration.
- Non-volatile acids, such as sulfuric acid and phosphoric acid, come from the metabolism of proteins and phospholipids.
- Bases primarily come from the bicarbonate ion (HCO₃⁻), which helps neutralize acids.
2. Buffers
The body uses buffers to maintain pH stability. A buffer system minimizes changes in pH by binding to or releasing hydrogen ions (H⁺). Key buffers include:
- Bicarbonate buffer system: The most important buffer in the blood, involving the equilibrium between carbonic acid (H₂CO₃) and bicarbonate (HCO₃⁻).
- Phosphate buffer system: Important in the regulation of pH in the kidneys and intracellular fluid.
- Protein buffers: Hemoglobin, albumin, and other proteins act as buffers in both the blood and tissues.
3. Regulation Mechanisms
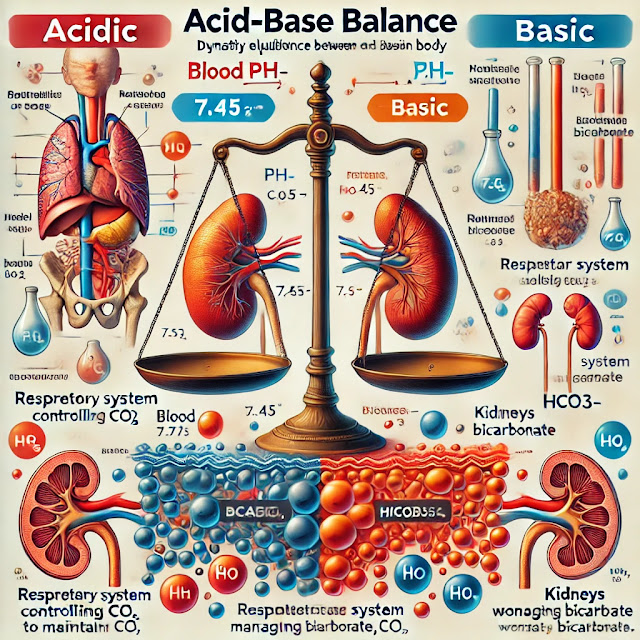
The body regulates pH through three main mechanisms:
- Respiratory Regulation:
- The lungs control the amount of carbon dioxide (CO₂) in the blood, which is a major determinant of acid levels.
- If blood pH drops (acidosis), the respiratory rate increases to expel more CO₂ (and thus reduce acidity).
- If blood pH rises (alkalosis), breathing slows down, retaining more CO₂, which converts to carbonic acid and lowers pH.
- Renal (Kidney) Regulation:
- The kidneys control pH by excreting hydrogen ions (H⁺) and reabsorbing bicarbonate (HCO₃⁻).
- They also produce new bicarbonate when necessary.
- This is a slower process compared to respiratory regulation but is more precise.
- Chemical Buffers:
- Immediate action from buffers in the blood, such as bicarbonate, proteins, and phosphates, helps prevent rapid pH changes.
4. Disorders of Acid-Base Balance
When the body cannot maintain pH within the normal range, acid-base imbalances occur. These can be divided into two broad categories, depending on whether the issue stems from metabolic or respiratory origins:
- Acidosis (pH < 7.35):
- Metabolic Acidosis: Results from the accumulation of acids (e.g., lactic acid, ketoacidosis in diabetes) or loss of bicarbonate (e.g., diarrhea).
- Respiratory Acidosis: Caused by hypoventilation (reduced breathing) leading to the retention of CO₂, which increases acid levels.
- Alkalosis (pH > 7.45):
- Metabolic Alkalosis: Often results from excessive loss of hydrogen ions (e.g., vomiting, use of diuretics) or gain of bicarbonate (e.g., antacid overuse).
- Respiratory Alkalosis: Results from hyperventilation (e.g., anxiety, high altitudes), which reduces CO₂ levels and causes a rise in pH.
5. Compensation Mechanisms
- Respiratory Compensation: For metabolic disorders, the lungs will adjust the breathing rate to compensate for pH changes. For example, in metabolic acidosis, hyperventilation helps reduce CO₂ and raise pH.
- Renal Compensation: For respiratory disorders, the kidneys will adjust hydrogen ion excretion and bicarbonate reabsorption to restore pH. This compensation takes hours to days.
6. Assessment of Acid-Base Balance
- Arterial Blood Gases (ABGs) are used to assess the acid-base status by measuring:
- pH: To determine if the blood is acidic or alkaline.
- PaCO₂ (partial pressure of CO₂): To assess respiratory involvement.
- HCO₃⁻ (bicarbonate): To evaluate metabolic involvement.
Importance of Acid-Base Balance
The maintenance of a stable pH is essential for:
- Enzyme function: Enzymes, which regulate almost every biochemical process in the body, work best within specific pH ranges.
- Electrolyte balance: Imbalances can affect potassium, calcium, and other electrolytes, leading to issues like muscle cramps, arrhythmias, or neurological symptoms.
- Oxygen transport: Hemoglobin’s ability to carry oxygen is influenced by blood pH.
In conclusion, acid-base balance is a complex and dynamic process that involves multiple organs and systems. The body has robust mechanisms to maintain this balance, but disruptions can lead to serious health issues.







0 Comments
Thanks for your feedback, i'll get back to you soon.